Acid Rain Formula And Air Pollution Problem
Acid rain is formed when certain gases dissolve in rainwater to form acids. Acid rain by definition in the Chemistry book states: Rainwater that has become excessively acidic because of absorption of pollutant oxides, notably S03, produced by human activities (1). There are airborne pollutants that cause acid precipitation for instance sulfur and nitrogen.
By far the most common cause of acid rain is sulphur dioxide, which is believed to cause 70 percent of acid rain, with various oxides of Nitrogen (NOX) causing the remaining 30 percent (4). An article in the New York Times is about the Federal Study of acid rain and its effects. This study shows that acid precipitation is a serious problem in the Adirondacks and is a growing threat to forests and watersheds (2). This study explains that Congress had made amendments to the Clean Air Act in 1990, that was supposed to cut the sulfur dioxide and nitrogen oxide emissions in half in coal-fired power plants and other industries(2).
Order custom essay Acid Rain Formula And Air Pollution Problem with free plagiarism report
 450+ experts on 30 subjects
450+ experts on 30 subjects
 Starting from 3 hours delivery
Starting from 3 hours delivery
The industries have problems disposing of these products properly because of the cost and difficulty of doing it. These industries must reduce their SO2 emissions by roughly 10 million tons annually and their NOX emissions by 2 million tons annually, by the year 2000 (3). These amendments are good and should help the pollution problem, but in the amendments the way to meet these goals is not provided. So because of this, these products are emitted into the atmosphere. The federal study also shows that acid precipitation is both more complex and intractable than had been thought 10 years ago (2).
These excess oxides are found from Colorado, to West Virginia, to Los Angeles, and most likely everywhere else in this world. Using natural gas can help to reduce the sulfur oxide emissions. Another way of reducing sulfur emissions into the atmosphere is by removing the sulfur on the oil and coal before burning it, which can also be very expensive(1). The study also shows that fish, insect and vegetation species have already been killed off as a result of decades of acid rain(2).
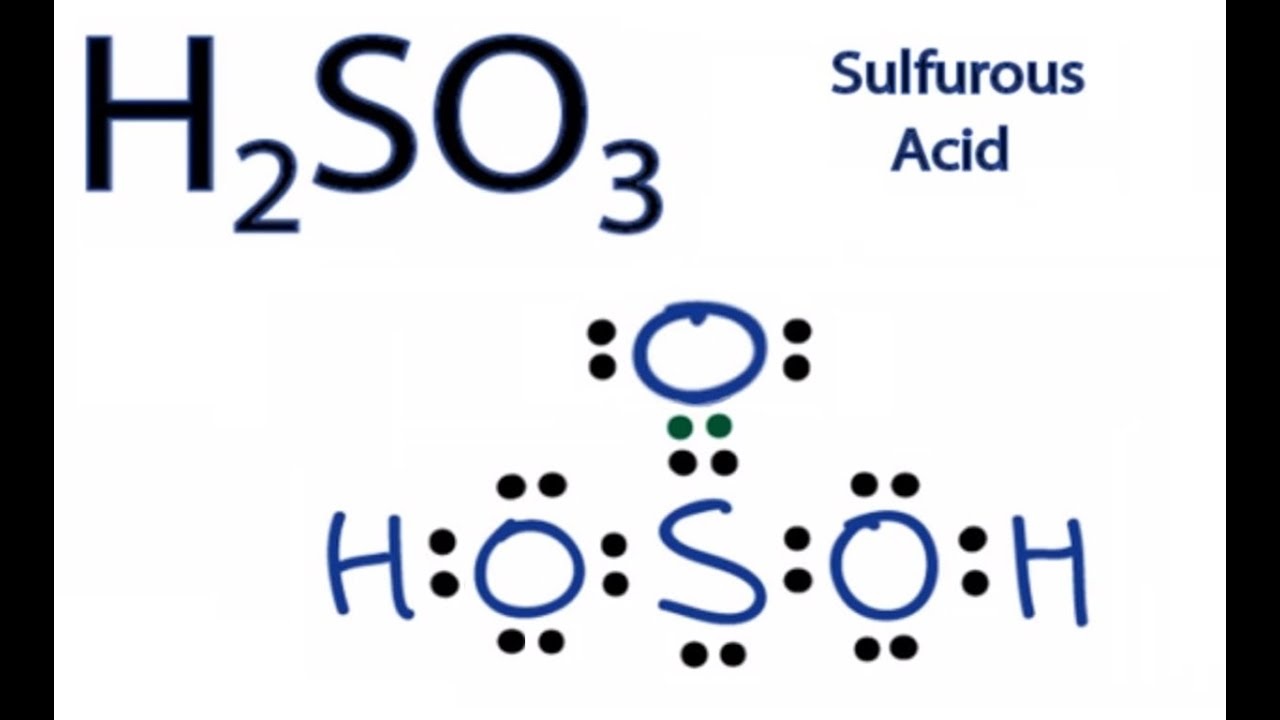
H2So3 Formula
After heavy rainfall, streams lack natural acid buffers will suffer sudden waves of high acidity that kill off fish larvae and eggs (2). Rainfall usually has a pH of 5, which suggests that it is naturally acidic(1). The pH scale levels go from zero to fourteen. A neutral solution has a pH of 7. Below the pH of 7, is an acidic solution and above the pH of 7, is a basic solution. Acid rain has a pH of 4, which indicates that it is more acidic than normal rainfall. At pH levels below 4.0 all vertebrates, most invertebrates, and many microorganisms are destroyed (1). It is not just water and animals that acid rain destroys. Acid rain also destroys metals and stone building materials. These reactions between water and sulfur dioxide and nitrogen oxides go as follows:
SO2(g) + 2H2O(0) 2H+(aq) + SO43-(aq) + H2(g) SO2(g) + H2O(0) H2S03 2NOX(g) + H2O(1) 2H+ + 2NO3
The sulfuric acid and nitrous acid are not formed until the gases in the atmosphere come in contact with the rain. By reacting sulfur dioxide with calcium oxide to form calcium sulfite is a way of preventing escape of sulfur dioxide form industrial operations. These rather dense gases alone can cause breathing difficulties if allowed to accumulate in the atmosphere (5). Calcium Carbonate can also react with the acid rain to produce carbonic acid which will eventually dissolve the marble in which calcium carbonate makes up.
Lakes and oceans do not become acidic overnight, it may take years or even decades for this to happen. Acid rain is not only in rain form it can also come from snow, mist or fog or anything else that falls from the sky. The metabolism and decomposition of lakes and other waterways also become affected and become slowed. It s beautiful, until you realize there s nothing growing on the rocks. And there s no insects (2). In the New York Times article, this quote is trying to reiterate the effect of acid rain of the waterways.
Acid lakes are crystal clear because many of the decomposers are dead and all the leaves and animals that are dead, fall to the floor. The acid rain phenomenon clearly indicates that air pollution is not always a local problem as sources of SO2 and NOX emissions may be located hundreds of miles from where deposition occurs (3). Seventy percent of sulfur dioxide emissions or more than 15 million tons a year of sulfur dioxide comes from Electric utility power plants(3).
Cite this Page
Acid Rain Formula And Air Pollution Problem. (2017, Feb 17). Retrieved from https://phdessay.com/acid-rain-formula-and-air-pollution-problem/
Run a free check or have your essay done for you


