Extraction of Trimyristin
Isolation of trimyristin from nutmeg
Purpose:
The purpose of this lab is to isolate and recrystallize trimyristin from nutmeg via a simple
Order custom essay Extraction of Trimyristin with free plagiarism report
 450+ experts on 30 subjects
450+ experts on 30 subjects
 Starting from 3 hours delivery
Starting from 3 hours delivery
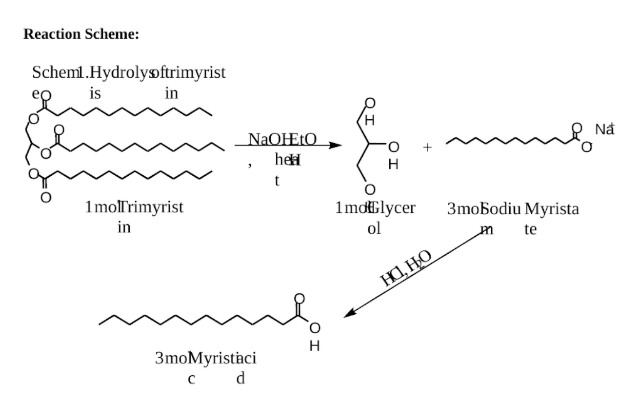
myristic acid, the fatty acid of trimyristin.
Reaction Scheme:
Scheml.Hydrolyoftrimyrist
Experimental Procedure:
1.025 g of nutmeg powder and 3 mL of TBME was added to a 10 mL round bottom flask. An air condenser was attached to the RB flask and the RB flask was gently heated until boiling, the mixture was boiled for 10 minutes and allowed to cool. The mixture was then filtered into a lean flask using a glass pipet with a small piece of cotton serving as a filter. After most of the product was filtered an additional 2 mL of TBME was added to the RB flask to wash any remaining product from the nutmeg powder, the additional product was then filtered and added
to the previously obtained product. The crude trimyristin (576 mg) was then recrystallized in 11.5 mL of acetone. The recrystallized trimyristin was collected via suction filtration and weighed. 60 mg of the refined trimyristin was added to a clean RB flask with 2 ml NaOH and 2 mL 95% ethanol. An air condenser was attached to the RB flask and the contents of the RB flask were gently refluxed for 45 minutes. The remaining refined trimyristin was recrystallized and collected as before. After reflux the contents of the RB flask were cooled to room temperature and poured into a beaker containing 8 mL H2O. 2 mL of HCL was then added to the beaker, dropwise, until no additional precipitate would form. The beaker was then cooled on ice and the precipitate was collected via suction filtration.
Results:
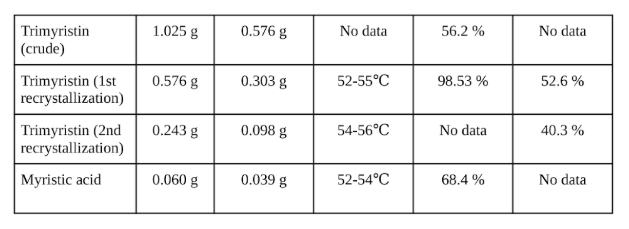
Percent recovery and yield is listed below for trimyristin and myristic acid. % recovery for each recrystallization was determined based on mass of starting material. % yield for the first recrystallization of trimyristin is based on 1.025 g of ground nutmeg, with a conservative estimate for trimyristin content of ground nutmeg of 30% based on numbers given by literature. % yield of crude trimyristin represents gross weight of product obtained and was not calculated based on 30% trimyristin content of ground nutmeg as it exceeds 30% of the starting mass.
Results are listed in Table 1 below.
Table 1. Trimyristin and Myristic acid isolation results
Discussion:
The extraction of trimyristin from nutmeg using TBME resulted in a 98.53% yield of trimyristin. The hydrolysis of trimyristin with NaOH and 95% ethanol resulted in a 68.4% yield of myristic acid. Melting point tests of the purified trimyristin was determined to be between 52-55°C for the first recrystallization and 54-56°C for the second recrystallization. Literature lists the melting point of trimyristin as 56-57°C. The 3°C range of the melting point (MP) for the first recrystallization suggests the the product obtained is of low purity as impurities in a solid will lower the melting point and result in a wider range for the MP range determined. The actual MP will likely be higher than the determined range, which matches the expectation given that the actual MP is between 56 and 57°C. The second recrystallization with a range of 2°C indicates that the product is sufficiently pure, though not completely pure. The MP range falls within the actual value for the MP of trimyristin given by the literature. The MP of the myristic acid obtained was between 52 and 54°C, this range of 2°C is an acceptable range for purity of the isolated product. Myristic acid has and actual MP between 54-55°C according to literature, the MP range of the product falls within this limit and therefore may be considered relatively pure.
Cite this Page
Extraction of Trimyristin. (2017, Jan 13). Retrieved from https://phdessay.com/extraction-of-trimyristin/
Run a free check or have your essay done for you


