Liquid Chromatography Essay
Chromatography is a separation technique in which the mixture to be separated is dissolved in a solvent and the resulting solution, often called the mobile phase, is then passed through or over another material, the stationary phase. The separation of the original mixture depends on how strongly each component is attracted to the stationary phase. Substances that are attracted strongly to the stationary phase will be retarded and not move alone with the mobile phase. Weakly attracted substances will move more rapidly with the mobile phase.
Liquid chromatography is an analytical technique that is useful for separating ions or molecules that are dissolved in a liquid phase. If the sample solution is in contact with a second solid or liquid phase, the different solutes will interact with the other phase to differing degrees due to differences in adsorption, ionic strength, polarity or size. These differences allow the mixture components to be separated from each other by using these differences to determine the transit time of the solutes through a column.
Order custom essay Liquid Chromatography Essay with free plagiarism report
 450+ experts on 30 subjects
450+ experts on 30 subjects
 Starting from 3 hours delivery
Starting from 3 hours delivery
Conventional Liquid Chromatography is most commonly used in preparative scale work to purify and isolate some components of a mixture. It s also used in ultra trace separations where small disposable columns are used once and then discarded. Analytical separations of solutions for detection or quantification typically use more sophisticated high-pressure liquid chromatography instruments.
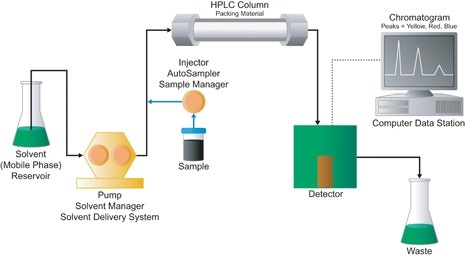
In liquid chromatography, the separator is called the column and consists in most cases of a tube filled with porous material called the stationary phase. A liquid, called the mobile phase, flows through the tube between the particles of stationary phase material. A liquid sample is taken from a mixture to be analyzed and introduced to a part of the system that is at elevated pressure. The sample is then transported to a separator by the flow in the system.
After the column the separated compounds enter the detector, which measures a physical or chemical property of each, now relatively pure, compound and creates a proportional electronic signal. By calibrating with a standard mixture of known compounds, the nature of the compound in the mixture can be elucidated. The quantity of the relevant compounds in the mixture can be calculated by integration of the signal.
The components in the sample become distributed differently between the mobile and stationary phases because they have different interactions (Physical and chemical) with each phase. You can select the nature and strength of the interactions by your choice of phases. The components move with different speeds through the column, depending on their affinity for the different phases, and so they elute from the column at different times: the retention times. By changing the composition of the mobile phase during the elution, its possible to analyze a wider variety of compounds in a given time than would be possible under constant conditions.
Chromatography is used in chemistry and biochemistry research analyzing complex mixtures, purifying chemical compounds, developing processes for synthesizing chemical compounds, isolating natural products, or predicting physical properties. It is also used in quality control to ensure the purity of raw materials, to control and improve process yields, to quantify assays of final products, or to evaluate product stability and monitor degradation.
In addition, it is used for analyzing air and water pollutants, for monitoring materials that may jeopardize occupational safety or health, and for monitoring pesticide levels in the environment.
Cite this Page
Liquid Chromatography Essay. (2020, Apr 21). Retrieved from https://phdessay.com/liquid-chromatography-essay/
Run a free check or have your essay done for you


