Prevention of Calcium Carbonate Precipitation in Synthetic Formation Waters
A new graduated table inhibitor for bar of Ca carbonate precipitation in man-made formation Waterss
Abstraction
In this probe, a new repressive chemical composing was developed. The made inhibitor is based on the aqueous solutions of oxiethilidendiphosphone acid, hydrochloric acid, ammonium chloride, polyethylene polyamine-N-methylphosphonic acid and isopropyl intoxicant to forestall the precipitation of Ca carbonate in a long clip operation of the well. The laboratory surveies of new inhibitor showed that the developed inhibitor has a greater suppression efficaciousness and continuance of desorption in comparing with the tried inhibitor SNPH-5312, which is widely used in the Fieldss in Russia to forestall the precipitation of Ca carbonate. The efficiency and corrosion aggressivity of new developed inhibitor were evaluated in three different man-made formation Waterss, which contained assorted ion concentrations and were disposed for precipitation of Ca carbonate.
Order custom essay Prevention of Calcium Carbonate Precipitation in Synthetic Formation Waters with free plagiarism report
 450+ experts on 30 subjects
450+ experts on 30 subjects
 Starting from 3 hours delivery
Starting from 3 hours delivery
Introduction
Huge sums of H2O are injected into the reservoir to keep the reservoir force per unit area at the needed degree, whereby, salt deposition occurs as a consequence of the H2O combination [ 1 ] . As the depletion of the oil field and its transportation to the late phase of development with increasing high H2O cut Wellss, scaling job is aggravated. Besides, there is the demand for backdown residuary oil, necessitating the usage of modern engineerings to better oil recovery, including physical and chemical exposure, which besides stimulates the deposition of salts. The chief grounds of deposition of salts are altering of thermobaric conditions in the procedure of production and the mutual exclusiveness of injected and formation Waterss [ 2 ] .
Inorganic salts deposition on the interior surface of oilfield equipment takes topographic point in the procedure of field development of production of moire oil. Salt precipitation occurs in all operation methods of Wellss, but the most negative effects of scaling occur during oil production by electric submergible pumps ( ESPs ) [ 3 ] . Intense deposition of Ca carbonate on impellers ESP is due to the flow temperature addition of produced fluids, which is caused by the heat emanation of runing the submergible motor. Along with salt deposition in Wellss, intense salt precipitation is observed in the wellspring, oil grapevine assemblage, metering devices and installations for the readying of oil and besides in reservoir force per unit area care systems [ 4 ] .
The procedure of precipitation of Ca carbonate occurs in three phases. In the first measure, ions of Ca combine with carbonate ions to organize the molecule. Following, molecules combine in microcrystals that serve as crystallisation centres for the reminder of the solution. Crystal aggregates grow and precipitate or attached to the walls of equipment at certain sizes [ 5, 6 ] . Calcium carbonate is found in the signifier of solid white crystals. Factors act uponing the formation of carbonate sedimentations include that formation H2O must be supersaturated with Ca, carbonate or hydrogen carbonate ions [ 7 ] .
All control engineering of grading is divided into bar and remotion of scaling. The most effectual method is chemical method of bar by utilizing scale inhibitors. Basic technologies of inhibitor injection are divided as follows: reagent bringing into the wellbore and into the formation. Dose into the well is carried out by agencies of batcher dosing into the ring, into a given point along the capillary and the periodic injection into the ring through collectors. Dose into formation is done through squashing scale inhibitor, injection via injection Wellss ( in force per unit area care system ) , add-on of inhibitor by proppant during fracturing ( ScaleProp ) and injection of the inhibitor with the fracturing fluid during fracturing ( ScaleFrac ) [ 8, 9 ] .
The intent of this work is increasing of operational efficiency of bring forthing Wellss by bar of formation of Ca carbonate in the downhole equipment, utilizing the developed inhibiting composing.
Methods
In the conducted research lab experiments for the readying of chemical solutions was used distilled H2O, in connexion with necessity to extinguish the influence on the belongingss of the composing and the consequences of experiments of ion finding, which were contained in different fresh H2O in assorted concentrations and ratios of their common concentrations. In the readying of look intoing composing harmonizing to the needed volume of the composing and concentrations of constituents, were weighed deliberate sum of H2O and reagents. Medical panpipes and high preciseness research lab balances were used for the exact values aˆ‹aˆ‹of the reagents multitudes.
Scale inhibitor should be to the full compatible with formation H2O without the precipitation formation while salvaging their belongingss [ 10 ] . For the analysis, man-made solution, the ionic composing of which is near to the composing of formation H2O, is prepared. Inhibitory belongingss mostly depend on the content of Ca in the formation Waterss. Therefore, the compatibility standards can be that if in the readying of the inhibitor solutions in the H2O with a certain content of Ca2+, turbidness is non observed within 24 hours, the inhibitor at a given concentration is considered compatible with the given H2O.
The prepared graduated table inhibitor should be more effectual and stable. The effectivity of the inhibitor is evaluated by its consequence on formation H2O or man-made theoretical account of H2O. Using theoretical accounts provides high truth measurings [ 11 ] . Determination of an inhibitor 's effectivity is made by appraisal of mass alteration of precipitation, which is formed in mineralized H2O in the presence of inhibitor with regard to H2O with no inhibitor [ 12 ] . Calculation of the protective consequence of an inhibitor is carried out harmonizing to the equation:
E % = ? 100( 1 )
? 100( 1 )
Where Tocopherol is the scale inhibitor efficiency, m0and m are the multitudes of salt precipitate in the H2O with inhibitor and without inhibitor in gm, severally.
The new graduated table inhibitor must hold the low corrosiveness. Corrosiveness of the developed composing is evaluated through the mass decrease of mention samples after their submergence in the inhibitor solution. Corrosion aggressivity of reagents was evaluated by hydrometric method - the weight loss of the samples. The corrosion rate of samples ( denseness of steel samples is 7821 kg.m-3) was calculated from the equation:
Voltdegree Celsiuss=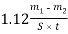 ( 2 )
( 2 )
Where Vdegree Celsiussis the corrosion rate of the used sample in mm/year, m1and m2are the mass of the metal samples before and after the trial in gm, severally, S is the surface country of samples in m2, t is the trial clip in hr.
Scale inhibitors should hold good adsorption-desorption features, heat opposition and minimum toxicity [ 13 ] . Evaluation of surface assimilation and desorption ability of suppressing composing is performed through research lab filtering of suppressing solutions for nucleus samples. Filtration surveies of developed inhibitor on nucleus samples are investigated by utilizing the setup FDES-645 ( Formation Damage Evaluation System ) . Reservoir temperature and force per unit area conditions are applied when utilizing this setup.
Result and treatment
The consequences of surveies to find the ionic composing of the man-made formation Waterss are shown in table 1.
Table 1. Characteristic of man-made formation Waterss
| Parameters | Man-made formation H2O | |||
| First | Second | Third | ||
| pH | 6.92 | 7.34 | 7.13 | |
| Density, kg.m-3 | 1012 | 1023 | 1018 | |
| Ion content, mg/l | HCO3- | 20654 | 16331 | 22784 |
| Carbon monoxide32- | 10895 | 15473 | 12871 | |
| Chlorine- | 240 | 508 | 372 | |
| Calcium2+ | 17194 | 21469 | 19836 | |
| Milligram2+ | 3489 | 5287 | 4173 | |
| Sodium+ | 1075 | 974 | 1248 | |
| K+ | 647 | 518 | 692 | |
| Entire dissolved salts, g/l | 54.19 | 60.56 | 61.98 | |
| Type of H2O harmonizing to the Sulin 's system | Chloride-calcium | Chloride-calcium | Chloride-calcium | |
Harmonizing to the categorization Sulin 's system, all man-made formation Waterss are a Ca chloride type. Sulin 's system is more descriptive of crude oil formation Waterss than are the other systems [ 14 ] . The entire mineralization of Waterss is located in the scope of 54 - 62 g/l. The theoretical accounts of H2O have the big concentration of hydrogen carbonate, carbonate and Ca ions, which are the chief factor of formation of Ca carbonate salt in the H2O because formation H2O must be supersaturated with these ions to precipitate this salt [ 15 ] .
The developed composing of inhibitor is evaluated by finding the residuary content of scale inhibitors in samples of liquid. The concentration finding of P of inhibitor in the formation H2O is based on the reaction of phosphate ion with molybdate in acerb medium [ 16 ] . The optical density ( optical denseness ) of the obtained solutions is measured by a exposure tintometer at length ?=540 nanometer in cells with an absorbing bed thickness of 30 millimeter. The optical denseness should non transcend one. Control sample is taken as a standard solution. Each sample is measured on photoelectrocolorimeter two or three times, the arithmetic obtained values are used for the consequence of measuring. From the obtained informations, a standardization curve is plotted by utilizing on the horizontal axis the concentration of inhibitor in mg/l, and on the perpendicular axis the magnitude of its matching optical denseness. As shown in figure 1, the ensuing values of the optical denseness are correlated with the standardization graph and the concentration of inhibitor is found in the trial solution as a consequence of the experiments.
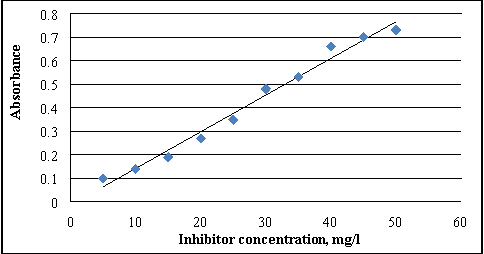
Figure 1. The alteration in optical denseness of the solution, depending on the content of the inhibitor in H2O
Evaluation of the effectiveness action of graduated table inhibitors by their ability to forestall the salt precipitation were carried out in the liquid solution of man-made formation Waterss. Trials were performed at a temperature of 25 ?C at the exposure clip of 24 hours. The consequences are presented in table 2.
Table 2. Evaluation of the effectivity graduated table inhibitors
| Scale inhibitor figure | Chemical composing of graduated table inhibitor | Scale suppression efficiency ( in 30 mg/l of inhibitor ) , % | ||
| First H2O | Second H2O | Third H2O | ||
| 1 | Oxiethilidendiphosphone acid 3 % , ammonium chloride 4 % , polyethylene polyamine-N-methylphosphonic acid 4 % , hydrochloric acid 10 % , isopropyl alcohol 2 % , H2O – balance | 91 | 90 | 92 |
| 2 | Oxiethilidendiphosphone acid 1 % , ammonium chloride 6 % , polyethylene polyamine-N-methylphosphonic acid 2 % , hydrochloric acid 5 % , isopropyl alcohol 6 % , H2O – balance | 89 | 87 | 88 |
| 3 | SNPH-5312, the composite reagent of P | 87 | 85 | 81 |
As shown in table 2, the consequences of the experiment revealed that the developed chemical composings have the necessary protective consequence ( effectivity of more than 85 % ) for Ca carbonate in dosing rate of 30 mg/l. The inhibitor figure 1 gives the higher effectivity for bar of Ca carbonate precipitation in all formation Waterss. The difference between the inhibitors figure one and two is the alteration in mass fraction of inhibitor constituents. Inhibitor SNPH-5312 is an industrial inhibitor for bar of Ca carbonate formation, which is used in oil field. This inhibitor is based on the composite reagent of P. Table 2 illustrates that SNPH-5312 can protect Ca carbonate formation up to 87 % .
Surveies have been conducted to find the compatibility of scale inhibitors with the formation Waterss. All inhibitors were compatible in three man-made formation Waterss, and the consequences showed all the above chemical composing can be prepared in the formation Waterss.
The usage of chemical reagents for forestalling the deposition of salts in the Wellss is associated with the usage of chemically aggressive environments. A scale inhibitor is anticorrosion if there is no opposing on the surface of the sample and corrosion rate does non transcend 0.1 mm /year. The caustic activity of above graduated table inhibitors was carried out by hydrometric method by utilizing metal home bases through soaking for 72 hours at 25 ?C.
Table 3. Consequences of probe of the corrosion rate of graduated table inhibitors
| Scale inhibitor | Test continuance, hour | First H2O | Second H2O | Third H2O | |||
| Mass decrease, g | Corrosion rate, mm/year | Mass decrease, g | Corrosion rate, mm/year | Mass decrease, g | Corrosion rate, mm/year | ||
| 1 | 72 | 0.0013 | 0.0404 | 0.0015 | 0.0467 | 0.0017 | 0.0529 |
| 2 | 72 | 0.0017 | 0.0529 | 0.0019 | 0.0591 | 0.0020 | 0.0622 |
| 3 | 72 | 0.0021 | 0.0653 | 0.0021 | 0.0653 | 0.0022 | 0.0684 |
From the informations in table 3, it can be noted that all the above chemical composings showed an allowable corrosion rate ( less than 0.1 mm/year ) . Therefore, these reagents can be considered as reagents to forestall grading in Wellss.
The initial concentrations of the reagents in suppressing composings are different, and so it is possible to compare the kineticss of the comparative concentrations of the solutions. The used nucleus samples had mean porousness of 20 % and permeableness of 70 mendeleviums. Figure 2 shows the consequences of finding of the comparative concentrations of the inhibitor reagents in the composings for the surface assimilation procedure at temperature of 120 ?C and force per unit area of 300 standard pressure. Laboratory surveies showed that the confining surface assimilation is achieved when pumping 14 pore volumes for suppressing composings figure 1 and 2, for complete surface assimilation of SNPH-5312, 15 pore volumes must be pumped. By comparing the comparative concentrations of reagents in figure 2 during surface assimilation, it can be concluded that the surface assimilation is faster when utilizing suppressing composings figure 1 and 2. Harmonizing figure 2, more unvarying surface assimilation is observed in the composing figure 1.
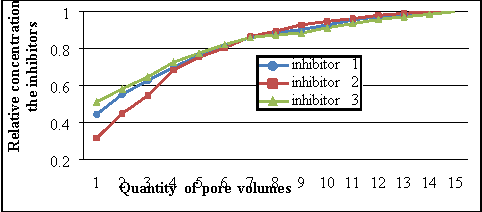
Figure 2. Concentration alterations of the inhibitor solution in the surface assimilation procedure in the nucleus
Once the nucleus left for 24 hours to find the surface assimilation equilibrium, formation H2O is pumped into the nucleus to displace suppressing composing. Consequences of finding of the comparative concentrations of inhibitors are shown during the desorption procedure in figure 3. The optimum and recommended concentration of oxiethilidendiphosphone acid in the composing for field conditions, is 10-15 mg/l, it corresponds to the comparative concentration of 0.0001. Harmonizing to figure 2, utilizing the inhibitor SNPH-5312 can supply the needed remotion of the inhibitor, which is sufficient for effectual protection of precipitation of Ca carbonate, when pumping through the nucleus sample of 27 pore volumes of H2O. Effective protection against formation of Ca carbonate under similar conditions persists in pumping 37 pore volumes of H2O when utilizing the developed suppressing composing figure 1, and 32 pore volumes of H2O by composing figure 2. This demonstrates that the developed composing have 1.37 times greater continuance of desorption in comparing with the inhibitor SNPH-5312.
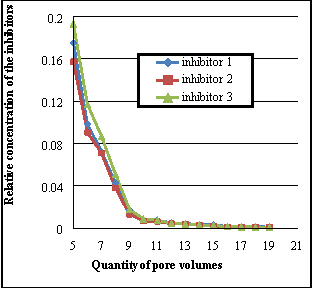
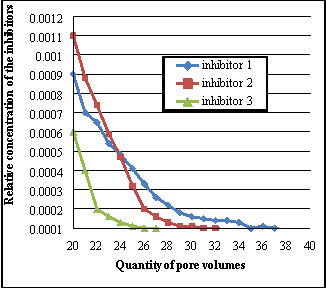
( a ) ( B )
Figure 3. Concentration changing of the inhibitor solution in the desorption procedure on the nucleus, ( a ) from 5 to 20 pore volumes, ( B ) from 20 to 40
Data analysis on remotion of considered repressive composings show that a important part of the free inhibitor ( non-adsorbed ) is passed in pumping the first two volumes of pore infinite. The efficiency of the developed composing is explained by the mechanism of influence on the stone acidic additives belonging to its composing.
Decisions
Inhibitory chemical composing was developed for the bar of deposition of Ca carbonate with an optimum ratio of constituent oxiethilidendiphosphone acid 3 % , ammonium chloride 4 % , polyethylene polyamine-N-methylphosphonic acid 4 % , hydrochloric acid 10 % , isopropyl alcohol 2 % , H2O – balance. The used graduated table inhibitor was evaluated in footings of influence on corrosion actions and it was in the scope of 0.040-0.053 mm/year when the maximal allowable rate is 0.1 mm/year. The new inhibitor was effectual for scale bar of Ca carbonate up to 92 % . The research lab surveies showed that the developed composings have about 1.37 times longer continuance in comparing with the desorption of the tried inhibitor SNPH-5312, which is widely used in the Fieldss for forestalling formation of Ca carbonate.
Mentions
- Chunfang Fan, Amy Kan, Ping Zhang, Haiping Lu, Sarah Work, Jie Yu, Mason Tomson. Scale Prediction and Inhibition for Oil and Gas Production at High Temperature/High Pressure. Society of Petroleum Engineers ( SPE ) 2012 ; 17 ( 2 ) : 379-392. Department of the interior: 10.2118/130690-PA
- J. Moghadasi, H. Muller-Steinhagen, M. Jamialahmadia, A. Sharif, M. Model Study on the Dynamicss of Oil Field Formation Damage Due to Salt Precipitation from Injection. Journal of Petroleum Science and Engineering 2004 ; 43 ( 3-4 ) : 201–217. Department of the interior: 10.1016/j.petrol.2004.02.014
- Neil Poynton, Alan Miller, Dmitry Konyukhov, Andre Leontieff, Ilgiz Ganiev, Alexander Voloshin. Squashing Scale Inhibitors to Protect Electric Submersible Pumps in Highly Fractured, Calcium Carbonate Scaling Reservoirs. Presented at the SPE Russian Oil and Gas Technical Conference and Exhibition28-30 October 2008 ; Moscow, Russia. ( in Russian ) . Department of the interior: 10.2118/115195-RU
- Mackay EJ. Scale Inhibitor Application in Injection Wells to Protect Against Damage to Production Wells: When does it Work. Presented at SPE European Formation Damage Conference 25-27 May 2005 ; Scheveningen, Netherlands. Department of the interior: 10.2118/95022-MS
- Mona El-Said, Mahmoud Ramzi, Thanaa Abdel-Moghny. Analysis of oilfield Waterss by ion chromatography to find the composing of scale deposition. Desalination 2009 ; 249 ( 2 ) : 748-756. Department of the interior: 10.1016/j.desal.2008.12.061
- Tomson, N.B. , G. Fu, M.A. Watson, A.T. Kan. Mechanisms of mineral scale suppression. Society of Petroleum Engineers ( SPE ) 2003 ; 18 ( 3 ) : 192-199. Department of the interior: 10.2118/84958-PA
- T. Kumar, S. Vishwanatham, S.S. Kundu. A research lab survey on pteroyl-l-glutamic acid as a scale bar inhibitor of Ca carbonate in aqueous solution of man-made produced H2O. Journal of Petroleum Science and Engineering 2010 ; 71 ( 1-2 ) : 1-7.s DOI:10.1016/j.petrol.2009.11.014
- Khormali A, Petrakov D. Scale Inhibition and its Effectss on the Demulsification and Corrosion Inhibition. International Journal of Petroleum and Geoscience Engineering 2014 ; 2 ( 1 ) : 22-33.
- Olesya Vladimirovna Levanyuk, Alexander M. Overin, Almaz Sadykov, Sergey Parkhonyuk, Bernhard R. Lungwitz, Philippe Enkababian, Alexander Vladimirovich Klimov, Sergey Legeza. A 3-Year Results of Application a Combined Scale Inhibition and Hydraulic Fracturing Treatments utilizing a Novel Hydraulic Fracturing Fluid, Russia. Presented at the SPE International Conference and Exhibition on Oilfield Scale 30–31 May 2012 ; Aberdeen, UK. Department of the interior: 10.2118/155243-MS
- Richard A. Dawe, Yuping Zhang. Dynamicss of Ca carbonate scaling utilizing observations from glass micromodels. Journal of Petroleum Science and Engineering 1997 ; 18 ( 3-4 ) : 179-187. Department of the interior: 10.1016/S0920-4105 ( 97 ) 00017-X
- Matty JM, Tomson MB. Effect of multiple precipitation inhibitors on Ca carbonate nucleation. Applied Geochemistry 1988 ; 3 ( 5 ) : 549-556. Department of the interior: 10.1016/0883-2927 ( 88 ) 90026-1
- Drela I, Falewicz P, Kuczkowska S. New rapid trial for rating of scale inhibitors. Water Research 1998 ; 32 ( 10 ) : 3188-3191. DOI:10.1016/S0043-1354 ( 98 ) 00066-9
- Ada Villafafila Garcia, Kaj Thomsen, Erling H. Stenby. Prediction of mineral graduated table formation in geothermic and oilfield operations utilizing the Extended UNIQUAC theoretical account: Part II. Geothermics 2006 ; 35 ( 3 ) : 239-284. Department of the interior: 10.1016/j.geothermics.2006.03.001
- A. G. Ostroff, Comparison of Some Formation Water Classification Systems, AAPG bulletin American Association of Petroleum Geologists, 1967 ; 51 ( 3 ) : 404-416.
- Chen T, Neville A, Yuan M. Calcium carbonate graduated table formation—assessing the initial phases of precipitation and deposition. Journal of Petroleum Science and Engineering 2005 ; 46 ( 3 ) : 185-194. Department of the interior: 10.1016/j.petrol.2004.12.004
- MacAdam J, Parsons SA. Calcium carbonate graduated table formation and control. Reviews in Environmental Science and Bio/Technology 2004 ; 3 ( 2 ) : 159-169. DOI:10.1007/s11157-004-3849-1
Cite this Page
Prevention of Calcium Carbonate Precipitation in Synthetic Formation Waters. (2018, Aug 29). Retrieved from https://phdessay.com/prevention-of-calcium-carbonate-precipitation-in-synthetic-formation-waters/
Run a free check or have your essay done for you


